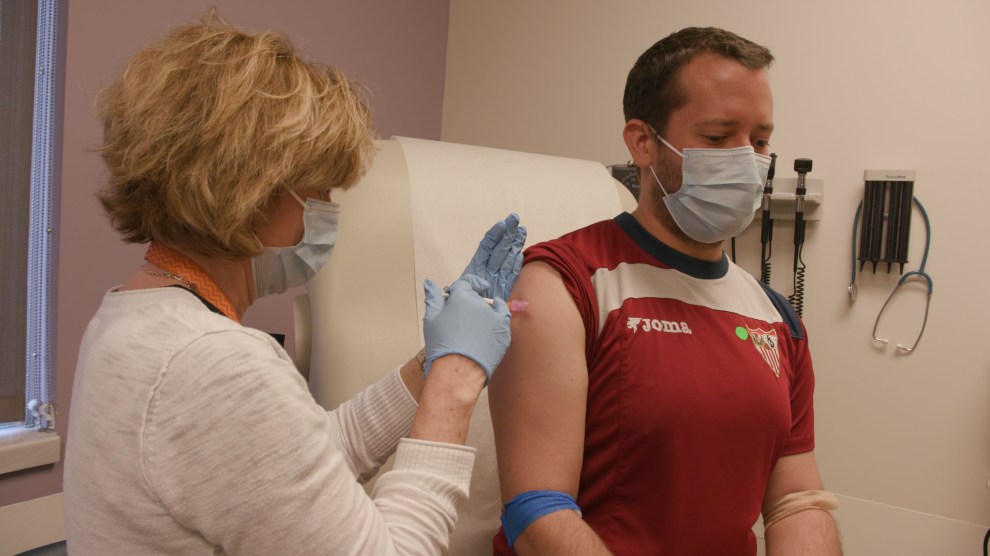
Sean Doyle receives a dose of the experimental coronavirus vaccine at Emory University. Damon Meharg/Emory University
Sean Doyle, a 31-year-old fourth-year med student at Emory University, is a vaccine trial veteran: Two and a half years ago, he volunteered at Emory to be injected with an experimental Ebola immunization for a trial that lasted 18 months. Last Friday, he headed to the lab once again, this time to receive a shot in the first US coronavirus vaccine trial. We talked to him about what it’s like to be a two-time vaccine guinea pig.
Read our interview with Dr. Evan Anderson, head of Emory’s coronavirus vaccine trial.
On what made him want to enroll: As a medical student right now, it’s a limited amount that we’re able to do—since we haven’t graduated and gone for medical training yet—to help with the response. So this seemed like a really great way to contribute to the cause.
I was contacted about being a participant in this clinical trial because I had been a participant for a vaccine trial that Emory had conducted for Ebola virus. Since I had completed that—and it had been a really rigorous trial—they thought that I might be a good candidate for this trial as well.
When Emory first reached out, I was really excited to participate, especially because I am a medical student and learned about vaccine development, and also about the potential benefit that these vaccines can have. What with everything that’s going on, it just seemed like a no brainer to get involved in this way.
On fears: No one is really sure how it’s going to behave when it’s put in your body. So some of my friends and family indicated a little bit of concern. But I’m familiar with the statistics on how rarely really severe reactions occur. So I wasn’t really that worried about being affected negatively by participating in this trial. It really does seem like the benefit would far outweigh any potential risks.
On not catching COVID-19 while he’s enrolled in the trial: I’m basically just taking the steps that everybody else is right now— socially isolating myself to the best I can. I do live alone, which makes it a little bit easier. I’m actually in a combined MD/PhD program at Emory. I’ve gone through the first half of medical training, and I’m currently PhD in cancer biology lab. So I’m working on writing. It keeps me busy at home, and I’m definitely not bored during my time here.
On getting the shot: I went through a screening process beforehand. I gave some blood samples, and they asked me a lot of questions related to my medical history that were kind of extensive. The screening process itself was not difficult or overbearing to go through.
I got the first vaccine last Friday. It was really just like getting any other vaccine, like a flu vaccine. There really wasn’t anything that was scary about it. It just felt like a very normal process to go through.
I have felt totally fine. The only thing that I noticed after getting the vaccine was that the site of injection had a little bit of tenderness for maybe like a day or two. Just like with any other vaccine that I had gotten.
On comparing this trial to the Ebola vaccine trial: To be honest, it hasn’t been quite as hard as the Ebola vaccine trial, which occurred over the span of about 18 months. This one is going to be about 12 to 14 months, I think. For the Ebola vaccine trial, they collected many more blood samples, and there were many more visits, in part because we received more vaccine doses during that trial.
The results have not been released yet because they are still doing some evaluation of the blood samples that were collected. But Dr. Anderson [the Emory physician who heads the coronavius vaccine trial] was also involved in that trial as well. And he told me that the results should be ready to be shared soon. So I’m very excited see what the results are going to be.
I haven’t experienced any side effects from the Ebola vaccines whatsoever.
On his hopes for the trial: I hope all of the folks who have enrolled will be able to complete it. I was told that the trial now is able to enroll more participants to cover folks that may represent a broader swath of the population and to further evaluate safety.
It will take a little bit to get to the point of determining its safety and efficacy. But this is a really important first step. And hopefully, it will lead to a good outcome.
This conversation has been edited for length and clarity.